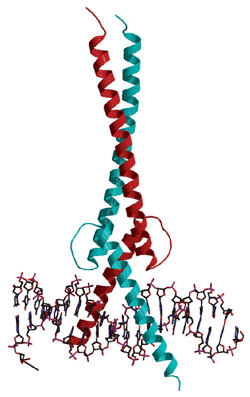
In the intricate realm of cancer research, one protein, MYC, stands out as a key player in facilitating the spread of 75% of cancers. While MYC is typically involved in healthy cell activity, it takes a treacherous turn when cancer cells emerge, contributing to their rapid growth and proliferation. The challenge lies in its elusive nature, being a shapeless protein with no clear structure that can be targeted by drugs effectively.
A team of researchers from the University of California, Riverside (UCR), however, might have unveiled a potential game-changer. Despite the difficulty posed by MYC’s lack of structure, these scientists have developed a peptide compound capable of binding and interacting with MYC, aiming to bring it back under control.
Biochemist Min Xue from UCR explains, “MYC is less like food for cancer cells and more like a steroid that promotes cancer’s rapid growth. That is why MYC is a culprit in 75 percent of all human cancer cases.”
The breakthrough hinges on the development of a peptide called NT-B2R, which proved highly effective in disabling MYC. The researchers, leveraging the limited structural insights into MYC, built a library of peptides, with NT-B2R emerging as a standout performer in tests using a culture made from human brain cancer cells.
In these tests, NT-B2R successfully bound to MYC, altering the regulation of many genes within the cells and ultimately reducing the metabolism and proliferation of the cancer cells. It’s akin to restraining the hands of a person, preventing them from engaging in harmful activities.
Critical to this success was the earlier recognition by the researchers that altering the structure and shape of peptides enhances their interaction with shapeless proteins like MYC. Xue explains, “Peptides can assume a variety of forms, shapes, and positions. Once you bend and connect them to form rings, they cannot adopt other possible forms, so they then have a low level of randomness. This helps with the binding.”
The researchers significantly improved the binding performance of NT-B2R, bringing it closer to their drug development goals. While these early results are promising, challenges remain. The current delivery method of the peptide via lipid nanoparticles needs refinement, as they are not ideal for drug dispensing.
Moreover, rigorous tests in human subjects are imperative before celebrating a definitive breakthrough. However, this discovery offers hope in addressing one of the ways cancer manipulates healthy biological processes for its survival.
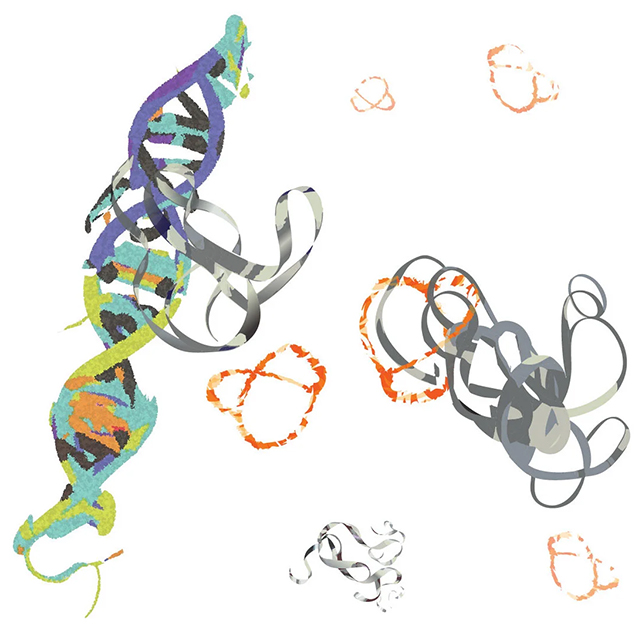
“Myoc represents chaos, basically, because it lacks structure,” says Xue. “That, and its direct impact on so many types of cancer make it one of the holy grails of cancer drug development. We are very excited that it is now within our grasp.”
References:
- Xue, M., et al. (Year of publication). “Title of the Research Paper.” Journal of the American Chemical Society, Volume(Issue), Page range.