In a groundbreaking discovery, researchers from the University Hospital Bonn have identified a crucial protein, EPAC1, that enhances the growth of beneficial brown fat cells. This finding holds the promise of revolutionizing weight-loss treatments by increasing brown fat mass and energy expenditure.
In a significant stride towards combating obesity and metabolic diseases, researchers from the University Hospital Bonn have pinpointed a pivotal protein—EPAC1—that plays a key role in enhancing the formation of beneficial brown fat cells. Brown fat, known for its ability to convert energy into heat, has been identified as a crucial factor in eliminating unwanted fat deposits and protecting against cardiovascular diseases.
The study, published in the journal Nature Cell Biology, sheds light on the potential of EPAC1 as a pharmacological target to increase brown fat mass and activity. Brown fat cells, often referred to as a “biological oven,” are essential for thermogenesis, a process vital for producing heat from blood sugar and fat molecules. This discovery opens up new avenues for developing medicines that support weight loss by promoting brown fat growth.
Lead author Prof. Alexander Pfeifer emphasizes the challenges of conventional weight-loss methods, stating, “Exercise and dieting are not enough to effectively and permanently shed the pounds.” The prevalent issue of obesity, characterized by an increase in white fat, poses a significant risk of cardiovascular diseases such as heart attacks and strokes. The goal of the researchers is to achieve additional energy release, addressing the limitations of traditional weight-loss approaches.
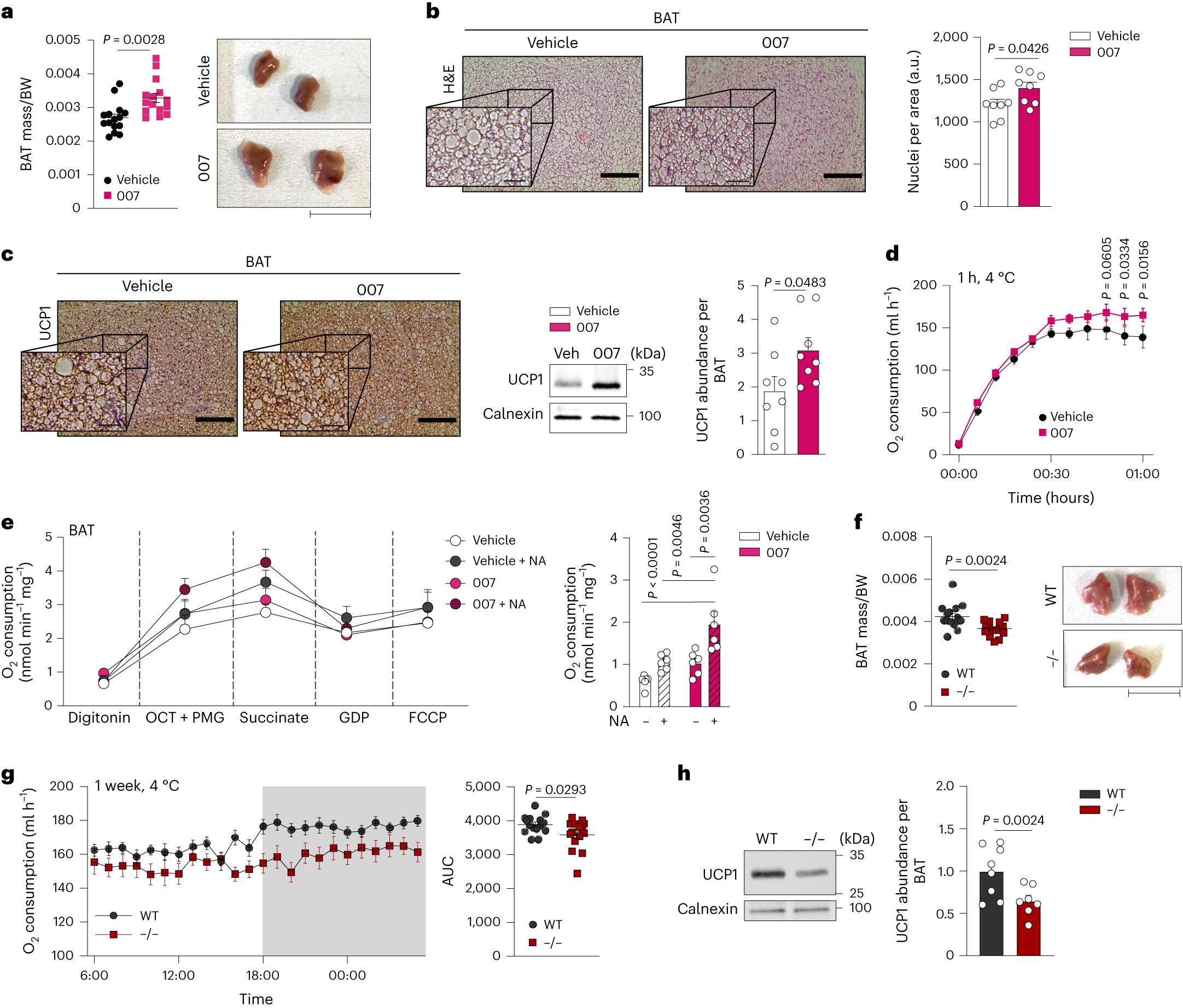
The research team, in collaboration with institutions such as the University Medical Center Hamburg-Eppendorf, Helmholtz Munich, and the University of Toulouse-Paul Sabatier, focused on investigating the cAMP signaling pathway in fat metabolism. Using a mouse model, they discovered the role of EPAC1 in the growth of brown fat cells, including its ability to stimulate the formation of “beige” cells in white fat.
Importantly, the study confirmed the presence of the cAMP signaling pathway and EPAC1 in human fat cells, showcasing the translational potential of the findings. The researchers also identified a non-functional human EPAC1 gene variant associated with an increased body mass index (BMI), highlighting the gene’s relevance in metabolic processes.
Prof. Pfeifer envisions the development of novel therapies targeting EPAC1 to increase brown fat mass, thereby enhancing energy expenditure. The study, conducted within the framework of the DFG Collaborative Research Center Transregio-SFB 333 “Brown and Beige Fat—Organ Interactions, Signaling Pathways, and Energy Balance,” contributes to a better understanding of adipose tissue types and their roles in metabolic diseases.
In summary, the discovery of EPAC1’s role in boosting ‘good’ brown fat formation presents a promising avenue for future weight-loss treatments. The potential to manipulate brown fat mass and energy expenditure through targeted therapies offers hope in addressing the global challenge of obesity and associated health risks.